Recent FDA Approvals & Designations Signal New Era for Cell Therapy Innovation
- Aug 11, 2025
- 4 min read
Updated: Aug 11, 2025
Published on celogics.com | Aug 12, 2025
The biotechnology landscape is experiencing a remarkable transformation, and the latest FDA approval data tells a compelling story of accelerated innovation and regulatory confidence in cell-based therapeutics. Over the past year, two groundbreaking cell therapy treatments have received FDA approval, representing not just individual victories for their respective companies, but a broader shift in how regulatory bodies approach these complex biological treatments.
Tecelra: Breaking the Solid Tumor Barrier

Tecelra represents a historic milestone as the first FDA-approved T cell receptor (TCR) gene therapy and the first engineered cell therapy specifically designed for solid tumors. The therapy targets melanoma-associated antigen A4 (MAGE-A4) in adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy.
Technical Innovation Tecelra modifies a patient's own T cells to recognize and attack the MAGE-A4 protein, designed as a one-time therapy. This represents the first engineered cell therapy approved for treating a solid tumor, as cell therapies previously reached patients primarily as treatments for blood cancers.
Clinical Impact The approval was based on results from the SPEARHEAD-1 (Cohort 1) trial, which included 44 patients. The accelerated approval pathway reflects the FDA's recognition of the significant unmet medical need in synovial sarcoma, a rare and aggressive cancer.
Zevaskyn: Revolutionary Cell Sheet Technology
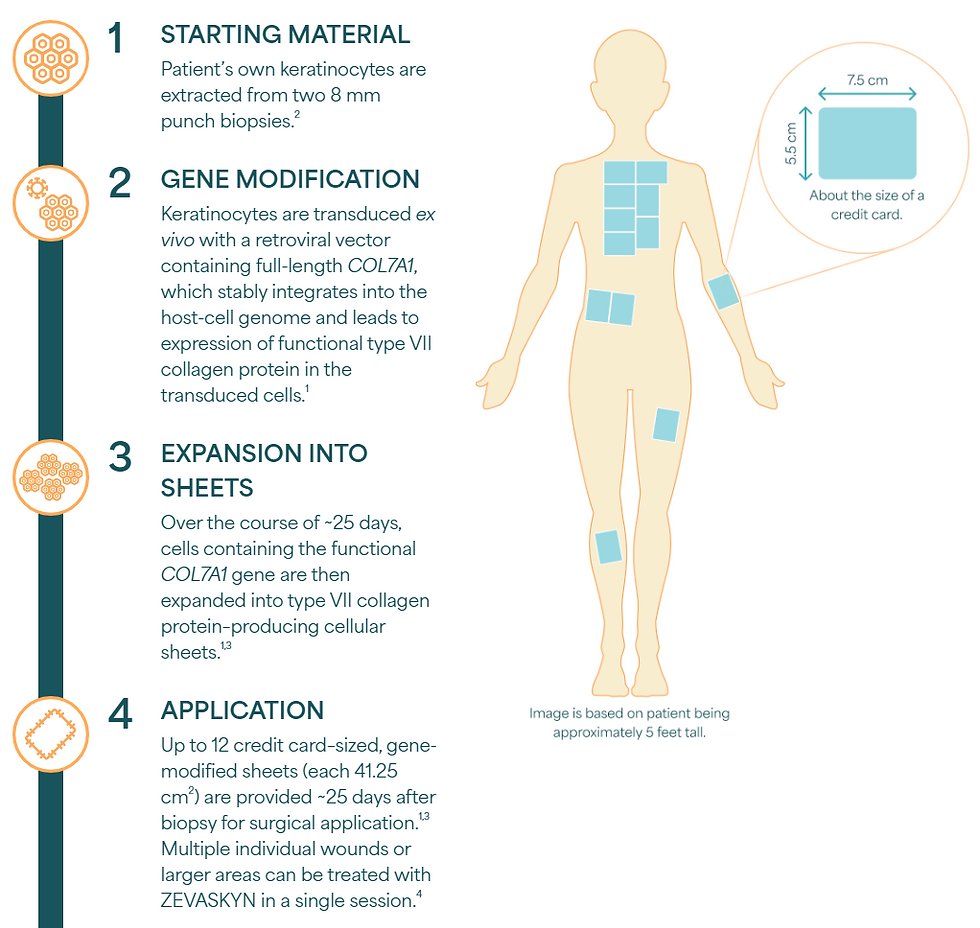
Zevaskyn is an autologous cell sheet-based gene therapy for treating wounds in adult and pediatric patients with recessive dystrophic epidermolysis bullosa (RDEB). This approval marks the third FDA-approved treatment and the first autologous, cell-based gene therapy for RDEB.
Novel Platform Approach Unlike traditional cell therapies, Zevaskyn utilizes a cell sheet-based platform that addresses the underlying genetic defect in RDEB patients. The FDA approval was based on the pivotal Phase 3 VIITAL study, a multi-center, randomized, intrapatient-controlled trial that met its two co-primary efficacy endpoints.
Market Access and Pricing Abeona priced Zevaskyn at $3.1 million, positioning it to compete with gene therapy from Krystal Biotech. The therapy is expected to become available in the U.S. starting in Fall 2025 through select Qualified Treatment Centers.
Regulatory Framework Evolution for Advanced Therapeutics
These approvals demonstrate the FDA's sophisticated approach to evaluating novel cellular platforms. Both Tecelra and Zevaskyn received accelerated approval pathways, reflecting the agency's commitment to bringing treatments for rare diseases and unmet medical needs to market more efficiently.
TCR Therapy Regulatory Precedent Tecelra's approval as the first FDA-approved T cell receptor (TCR) gene therapy establishes important regulatory precedent for future TCR-based treatments. The FDA's evaluation process for TCR therapies differs significantly from CAR-T approaches, requiring new safety and efficacy frameworks.
Cell Sheet Technology Assessment Zevaskyn's approval as an autologous cell sheet-based gene therapy required the FDA to develop evaluation criteria for this novel platform, setting the stage for future cell sheet-based therapeutics.
Accelerated Approval Pathways Both approvals utilized accelerated approval pathways, notably the Regenerative Medicine Advanced Therapy (RMAT) deignation, demonstrating the FDA's willingness to expedite promising treatments for serious conditions with limited therapeutic options.
Implications for the Biotech Industry
For Companies Developing TCR and Novel Cell Platforms
The success of Tecelra validates investment in T cell receptor technologies and solid tumor applications. Companies developing TCR-based approaches now have a regulatory roadmap and proof-of-concept for FDA approval in oncology applications.
For Advanced Therapeutic Platform Developers
Zevaskyn's approval opens new possibilities for companies working with cell sheet technologies, tissue engineering, and other advanced cellular platforms beyond traditional cell suspensions.
Market Access and Commercial Viability
The 30% reduction in manufacturing costs addresses one of the primary barriers to widespread cell therapy adoption. Healthcare systems have struggled to provide coverage for treatments costing $300,000-$500,000 per patient. These manufacturing improvements, combined with expanded patient populations, create a more sustainable commercial model.
Technology Trends Driving Success
Automation and Standardization
The successful companies have invested heavily in automated manufacturing systems that reduce human error, improve consistency, and lower labor costs. These systems also enable the scale-out manufacturing models necessary for global commercialization.
Next-Generation Cell Engineering
Recent approvals showcase more sophisticated cell engineering approaches, including:
Enhanced safety switches to mitigate adverse events
Improved persistence and trafficking capabilities
Reduced immunogenicity through advanced gene editing techniques
Supply Chain Innovation
Companies are developing distributed manufacturing networks that bring production closer to patients, reducing logistics costs and improving product viability upon delivery.
Looking Forward: What This Means for Innovation
The current approval environment suggests several key trends for the cell therapy sector:
Accelerated Development Timelines With clearer regulatory pathways and reduced approval times, companies can plan development programs with greater confidence in timelines and resource requirements.
Expanded Therapeutic Applications Success in traditional oncology applications is driving investment in cell therapies for autoimmune diseases, regenerative medicine, and other therapeutic areas.
Manufacturing as Competitive Advantage Companies that can demonstrate robust, scalable, and cost-effective manufacturing will have significant competitive advantages in bringing products to market.
Celogics' Perspective
At Celogics Inc., we view these developments as validation of our strategic focus on developing next-generation cell therapy platforms. Our research programs are designed to address the remaining challenges in the field while building on the regulatory and manufacturing innovations demonstrated by these recent approvals.
The regulatory environment's evolution creates opportunities for innovative companies to bring meaningful treatments to patients more efficiently than ever before. We're particularly encouraged by the FDA's demonstrated willingness to work with companies developing novel approaches that could further improve patient outcomes and accessibility.
Conclusion
The three recent FDA approvals represent more than individual product successes—they signal a maturing industry with established regulatory pathways, proven manufacturing solutions, and growing commercial viability. For patients, this means faster access to potentially life-saving treatments. For the biotech industry, it means a more predictable and efficient development environment.
As we look toward the remainder of 2025, we anticipate continued momentum in cell therapy approvals, with an expanding range of therapeutic applications and increasingly sophisticated treatment approaches. The foundation has been laid for cell therapy to become a standard component of modern medicine, moving beyond the experimental therapies of just a few years ago to established treatment options for patients worldwide.
The future of personalized medicine isn't just approaching—it's here, and the regulatory and commercial infrastructure to support it is stronger than ever.
For more insights on cell therapy development and the evolving biotech landscape, follow Celogics Inc. on LinkedIn and subscribe to our monthly research newsletter.
Tags: Tecelra, Zevaskyn, TCR Therapy, Cell Sheet Technology, FDA Approval, Solid Tumor Cell Therapy, Rare Disease Treatment, Gene Therapy


Comments